福可苏®——全球首款全人源CAR-T疗法,致力于为复发/难治性多发性骨髓瘤患者带来治愈的曙光,助力患者实现快速、深度、持久的缓解!
我们深知,在治疗过程中,关爱与陪伴同样至关重要。i-Connection将为您提供全方位的支持与保障,携手共度这段充满挑战的治疗旅程!


 1
2
3
4
5
1
2
3
4
5

 1
2
3
4
5
1
2
3
4
5

 1
2
1
2

 1
2
1
2

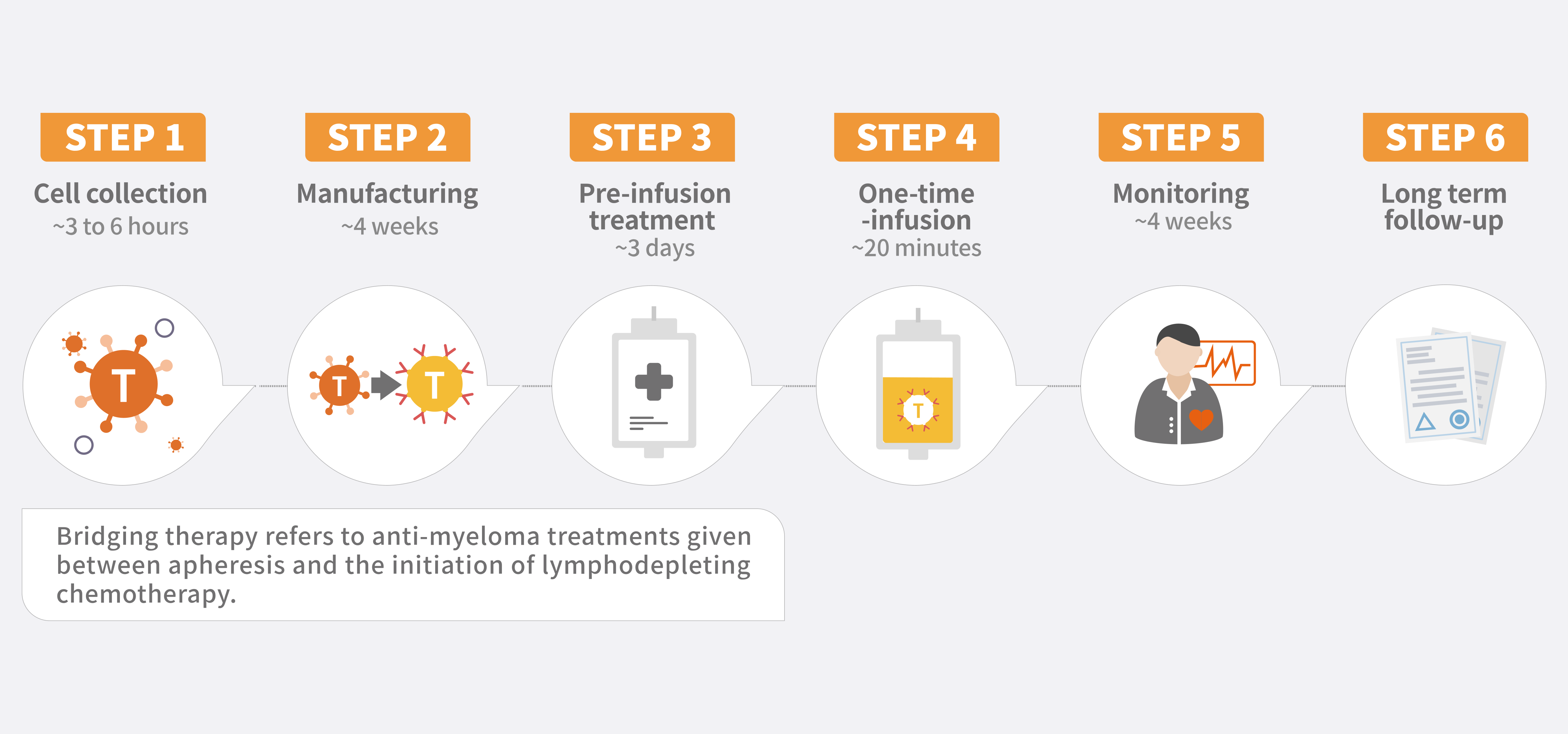
福可苏®与传统的癌症治疗方法(如化疗、放疗等)有显著区别。该疗法利用从您自身血液中提取的T细胞,通过基因改造将靶向B细胞成熟抗原(BCMA)的嵌合抗原受体(CAR)基因整合到分离出的T细胞中。 这一创新方法使得福可苏®能够准确识别和攻击体内的多发性骨髓瘤细胞,提供了独特的治疗途径。
作为一种定制化治疗,您需要前往获得认证的治疗中心,由专业医疗团队为您开具处方并进行药物输注。您的医疗团队将全程协助您进行详细的咨询和评估,以帮助您了解是否适合接受福可苏®治疗。
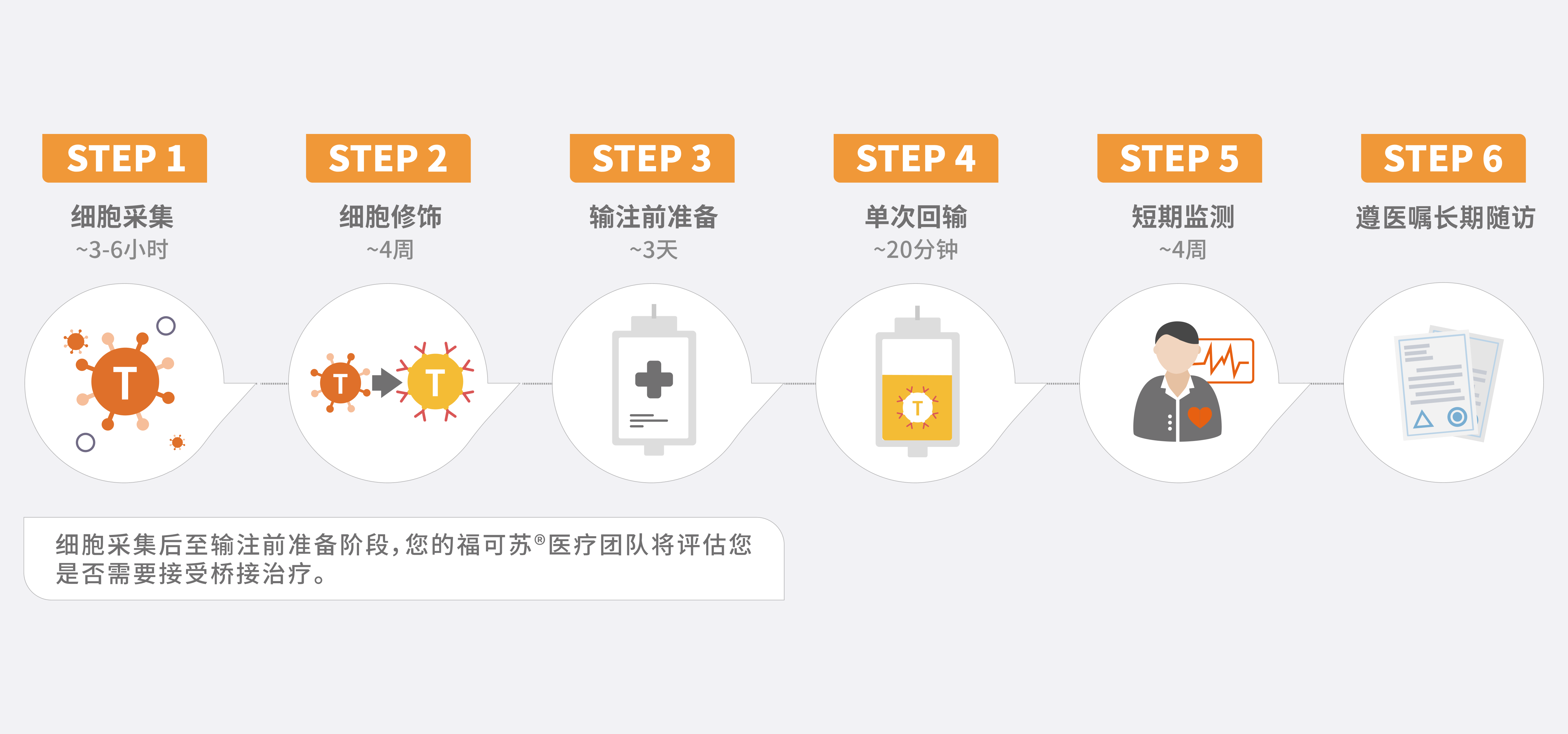
福可苏®与传统的癌症治疗方法(如化疗、放疗等)有显著区别。该疗法利用从您自身血液中提取的T细胞,通过基因改造将靶向B细胞成熟抗原(BCMA)的嵌合抗原受体(CAR)基因整合到分离出的T细胞中。 这一创新方法使得福可苏®能够准确识别和攻击体内的多发性骨髓瘤细胞,提供了独特的治疗途径。
作为一种定制化治疗,您需要前往获得认证的治疗中心,由专业医疗团队为您开具处方并进行药物输注。您的医疗团队将全程协助您进行详细的咨询和评估,以帮助您了解是否适合接受福可苏®治疗。

Equecabtagene autoleucel (eque-cel), also known as FUCASO, was developed by IASO Bio and was the first approved BCMA CAR-T cell product in China for treatment of patients with multiple myeloma.
FUCASO demonstrated consistent and reliable clinical potency, efficacy, and safety in the targeted population. It can induce fast, durable, and deep response to ensure prolonged survival benefits with manageable toxicities. We are hoping FUCASO would help patients blow away the obscured clouds of disease in the blue sky of their meaningful life in the near future.


 1
2
3
1
2
3

 1
2
3
1
2
3

 1
2
1
2

 1
2
1
2